Over 25 years experience in Cattle Embryo Transfer.
- Coolana
- Pine Creek
- Wattletop
- Eastern Plains
- Bald Blair
- Nairn Park
- Strathewen
- Anvil
- Jade Park
- Delamere
- Global Reproduction Solutions
- Beefmark Breeding Centre
- Avignon
- Cudgegong Park
- Lancaster
- Wombramurra
- Waitara
- Cobrabold
- Nairn Park
- Tycolah
- Longford Station
- Lake Wagyu
- Julianpete
- Wakefield
- La Frontiere
- Premier
- Sedalia
- Yancowinia
- A Bar Dot Genetics
- Bald Ridge Cattle
- Genetic Edge Australia
- Moombi
- Magnet
- Yamburgan
- Manchee Cattle Company
- Dunbeacon
- Chucklebud Cattle Company
- Chateau
- Oasis Collection Centre
- Winchester
- Trentbridge
- Weebollabolla
- Greenhills
- Nagol Park
- Nero
- Charleroi
- Kalinka
- Mawarra
- Birubi
- Booragul
- Eaglehawk
- High Range
- Waratah
- Langi Ghiran
- Fernleigh
- Summit
- Waterford
- The Hills
- Kevlynn Downs
- Wanura
- Merawah
- Kilburnie
- Academy
- Flemington Park
- 4-Ways
- Tarralaeha
Cattle Embryo Transfer
Australian Animal Genetics has been associated with embryo transfer in cattle for the past 25 years and has a large, diverse client base around Australia. AAG works with beef and dairy cattle and has assisted in introducing new breeds and genetics into Australia. AAG also has a dynamic export arm which has been involved in the export of superior Australian genetics around the world. Within our client base we can provide a service to aid the exchange or introduction of new genetics to existing or new clients.
AAG provides a full range of services in cattle embryo transfer. These include embryo collection and transfer and embryo freezing using either conventional or quick thaw freezing protocols.
AAG conducts embryo programmes on farm or in alliance with a number of embryo transfer centres. centres are based in Victoria (Oasis Collection Centre), NSW (Bovine Breeders, Armidale, Genetic Edge, Tamworth and Beefmark (Glen Innes) and Qld (ABar dot, Texas).

We offer:
-
Facilities
Minimum facilities required for embryo transfer
As cattle are handled intensively throughout the embryo programme, good cattle handling facilities are required. These consist of holding yards, a race and an effective crush for restraining animals during embryo collection and transfer. If animals cannot be adequately restrained flushing may be ineffective and trauma may be done to the uterus. During transfer damage can be done which interferes with the establishment of pregnancy. Keeping animals stress free throughout the hormone treatment programme and during the collection and transfer process is very important as stress can have an negative affect on results. Feeding animals in the yards after treatment may make them more amenable to the treatment regime.
The crush must be protected from direct sunlight as UV rays can harm embryos. Rain can lead to damage and breakdown of the sterility of equipment used for embryo collection and transfer. A solid roof or tarpaulin should be provided.
A table or bench for laying out of equipment beside the crush is essential. Rubbish bins should be provided for the collection of used disposable equipment.
The laboratory for embryo handling should be clean and dust free. In winter it should be capable of being warmed and in summer cooling may be required. Embryos survive best when the temperature is between 15 and 30 C. On some farms a purpose built laboratory is provided. This should be cleared of chemicals and be clean embryo collection day. Dust and dirt can contaminate the embryo collection dishes and this can lead to poor results. When a laboratory is not available, a table in the kitchen or dining room of the farm can be used. If the house is used, the table should be out of the way of routine family life. Embryo work requires concentration and attention to detail. Mistakes can be made if the technicians are distracted. A table set up in an open shed or outdoors is unacceptable as dust and sunlight can kill or contaminate the embryo holding solutions. ET technicians need to attempt to operate in sterile conditions.
-
Embryo Collection
Collection of embryos from the donor
Donors selected for ET are given a series of hormone injections to induce superovulation. These are done at a precise time of the cycle. The ET technician provides a hormone treatment timetable and this will provide dates and doses for the different hormones used in the programme.
A typical programme would be as follows:
An injection of prostaglandin [Estroplan, Lutalyse] is given, followed by the insertion of an intravaginal device (CIDR, Cue Mate) and then starting on day 14, twice daily [morning and evening] injections of Follicle Stimulating Hormone [FSH - Folltropin or Pluset] are given for four days. The donor is brought into heat by another injection of prostaglandin on day 16. When she comes into heat she is joined by AI or mated naturally. When using AI, it is important to watch the donor closely to detect the onset of standing heat. AI is done 10-12 hours after the first signs of standing heat and then again 12 hours later. If rare or expensive semen is being used, a single straw can be given around 18 hours after the first observation of standing heat. Embryo collection is done seven days later.
-
Donor Program Example
An Example of a Donor Program
DAY DONOR Treatment 1 Inject PG (am) 5 Insert CIDR (Progesterone implant) 14 Inject FSH (AM & PM) 15 Inject FSH (AM & PM) 16 Inject FSH (AM & PM)+ Inject PG (AM & PM)+ Pull CIDR 17 Inject FSH (AM & PM) 18 Heat Check / AI / Mate 19 Heat Check / AI / Mate 25 Flush Date Use the ET Program Planner to calculate important dates for your cattle ET Program.
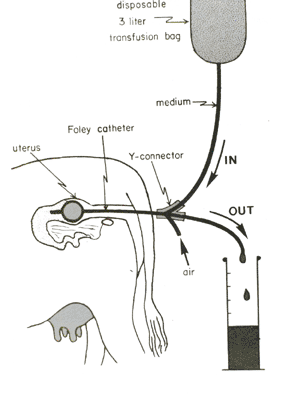
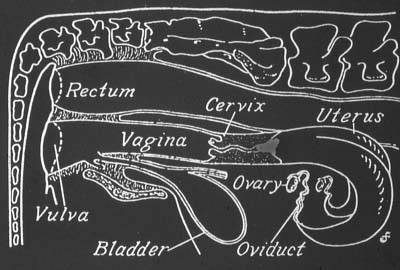
Embryos are flushed from the uterus using a catheter (see diagram). The catheter is passed through the cervix into the uterine horn to a precise position. It is held in place by the inflation of a small balloon [cuff]. A sterile buffered salt media is then washed through to uterus to dislodge the embryos. They are collected into a sterile embryo filter. The filter is then transferred to the laboratory and the embryos located using a stereomicroscope.
Embryos recovered, can be transferred fresh to suitable recipients, or frozen for later use. Provided they are stored properly in a well maintained liquid nitrogen tank, they have an infinite life span.
-
Donor Selection Criteria
Donor Selection Criteria
Donors are usually selected on the basis of superior genetic merit compared with the rest of the herd. Fertility is extremely important, so the ideal donor should be reproductively sound and must be cycling. Maiden heifers and cows can be used as donors. Cows should have calved at least 56 days and be cycling regularly before they are put into a flushing program.
Maiden heifers should weigh a minimumof 350 kg, be cycling and have a reproductive tract large enough to accommodate the flushing catheter. If there is any doubt about the suitability of an animal for flushing, contact your ET technician and have the animal examined by the technician or your local veterinarian.
-
Transferring Embryos
Transferring embryos to recipients
Recipients have their cycle manipulated by hormone treatments so they will be at the correct stage of their cycle to receive an embryo. There are several ways of achieving this with the aim being to have the recipient at the same stage of her cycle as the donor at the time of embryo collection. An example of one method of synchronisation using a CIDR implant and prostaglandin is shown below:
-
Recipient Program Example
An Example of a Recipient Program

Day Recipient Treatment 0 Insert CIDR + CIDIROL 7 Inject PG (AM) 8 Pull CIDR’s 9 Heat Check 10 Heat Check 11 Heat Check 17 Transfer Day Use the ET Program Planner to calculate important dates for your cattle ET Program.
 Recipients need to be checked for the onset of heat. This is important in determining their suitability to receive an embryo. Only animals that have been observed to be standing heat can be used for transfer. Transfer of the embryo is performed 7 days after heat. Implantation can be done surgically or non-surgically. AAG routinely implants non- -surgically but does surgical transfers if requested by the client. In our opinion there is no difference in the results achieved using non-surgical or surgical implants.
Recipients need to be checked for the onset of heat. This is important in determining their suitability to receive an embryo. Only animals that have been observed to be standing heat can be used for transfer. Transfer of the embryo is performed 7 days after heat. Implantation can be done surgically or non-surgically. AAG routinely implants non- -surgically but does surgical transfers if requested by the client. In our opinion there is no difference in the results achieved using non-surgical or surgical implants. -
Recipient Selection Criteria
Recipient Selection Criteria
Recipients should be reproductively sound with no history of infertility. Culls or animals that have failed to conceive under normal breeding conditions should NOT be used as recipients. Prior to the start of the programme they should be injected with Vitamins A, D & E and ” MULTIMIN”. They should aslo be vaccinated with “PESTIGUARD”.”".
Heifers can be used as recipients but they must weigh at least 350 kg and cycling before they can be included in a program. It is essential that embryos from high birth weight bulls are not implanted into heifers.
It is common for stud breeders to use the lower end of their herd for recipients as this eliminates the need to introduce animals of unknown disease status into their herds. When buying in recipients, attempt to ascertain their disease status and fertility. Introduced animals should be quarantined for a minimum of 30 days during which time they should be wormed, vaccinated and given injections of Vitamins A, D &E, MULTIMIN and PESTIGUARD. Any other procedure such as de-horning or should be done eight weeks before,or eight weeks after transfer.
Breeders taking part in the Johne’s disease market assurance programs can only introduce animals of equivalent or higher disease status into their herd.
-
Management for Optimum Results
Management Factors Involved in Achieving Optimum Results with Donors and Recipients
 Mineral deficiency is widespread in Australia and can lead to reduced fertility. If the mineral status of the herd is unknown animals should be treated with multi mineral supplement containing copper, selenium and cobalt. These are available as licks, boluses and injection. We recommend injecting with MULTIMIN as the best way of ensuring optimal levels of these minerals. Licks can cause under or over dosing depending on whether the animal is an aggressive or shy feeder. These and treatments such as 7 in 1 vaccine, PESTIGUARD and treatment for external and internal parasites should be given at least two months prior the programme.
Mineral deficiency is widespread in Australia and can lead to reduced fertility. If the mineral status of the herd is unknown animals should be treated with multi mineral supplement containing copper, selenium and cobalt. These are available as licks, boluses and injection. We recommend injecting with MULTIMIN as the best way of ensuring optimal levels of these minerals. Licks can cause under or over dosing depending on whether the animal is an aggressive or shy feeder. These and treatments such as 7 in 1 vaccine, PESTIGUARD and treatment for external and internal parasites should be given at least two months prior the programme.Body condition is important and animals that are too thin or too fat do not respond well to superovulation or synchronisation. As a general rule, animals should be in the condition that would give high conception rates under natural mating conditions. Best results are obtained when animals are on a rising plane of nutrition and gaining weight. The diet to achieve this depends on many factors such as the breed, age and stage of lactation of the animal.
Nutritional requirements can be met in a number ways. If pasture is of good quality and in plentiful supply the nutrient requirement may be met by pasture alone. Pastures that are predominantly clover or lucerne should be avoided as these may contain plant oestrogens which interfere with normal hormonal patterns and result in cystic ovaries or unfertilized or poor quality ova. Where lush pasture or pasture with a high content of clover or lucerne is fed; animals must have access to roughage such as oaten hay, straw or stalky pasture hay. Roughage slows the passage of food through the intestine allowing better absorption of nutrients. Other supplementary feeding may be required. Up to 2 kg of grain can be fed per day keeping in mind that it is essential to introduce ruminants to grain gradually to avoid acidosis. At all times the diet should contain adequate amounts of roughage.
Cows being flushed a number of times should be “let down” in condition immediately after the flush and then introduced back onto the feed one to two weeks before the start of the next program.
Flushing and transfer costs are considerable due to the price of drugs, semen and technical fees. The cost of feeding through the programme is justified by the return achieved through the good embryo production and high pregnancy rates after transfer.

